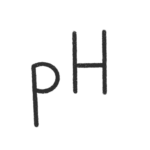
In most media for mammalian cells the pH is adjusted by a bicarbonate CO2 buffer system. The correct pH is essential for an optimal cell viability and cell growth. Cells are producing organic acids as metabolites which will reduce the pH in the cell culture medium. Even, if cells can tolerate a pH shift to lower values it is good to keep the pH as constant as possible. In the starting phase of a culture and for cells with low metabolic activity there is a risk of a pH shift to higher values, if the CO2 concentration is too low. This can kill your cells and need to be avoided. But what is the correct CO2 concentration for my cells? pH 7.0 – 7.2 is fine for most cell lines but have a look in the documentation for your cells. The only thing you need else is the bicarbonate (NaHCO3) concentration of your culture medium. Have a look at the formulation provided by the supplier. Once you have calculated the correct CO2 concentration, adjust the CO2 setpoint in your incubator. You would like to know how the values are calculated? – Have a look at the equation. CO2 = CO2 concentration in [%] pH = pH needed for your cells c(NaHCO3) = bicarbonate concentration [g/L] M = molar mass of NaHCO3 (=84.01 [g/mol]) pKs = dissociation constant L = solution coefficient of the bicarbonate buffer system (= 2.38e-7) a = conversion factor of partial pressure in [mmgH] to [%] (0.133322) CO2 calculator
(pH in a bicarbonate buffered system)
![]()